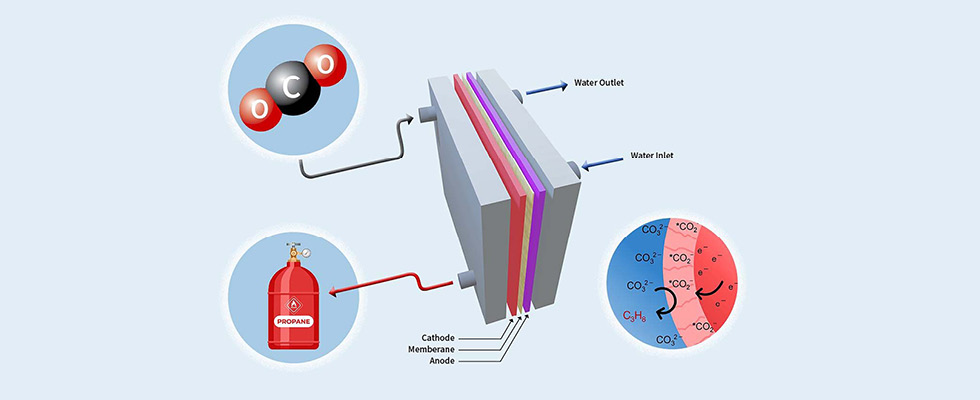
In the push for cleaner energy sources, and as the United States works toward its goal of net-zero greenhouse gas emissions by 2050, what better solution is there than taking carbon dioxide — the primary greenhouse gas causing climate change — and converting it into propane in a way that is both economically viable and scalable across many different use cases?
In recently released research in the form of a paper co-authored by Assistant Professor of Chemical Engineering Mohammad Asadi (Illinois Institute of Technology, or Illinois Tech), the team accomplishes just that: the breakthrough of an electrolyzer device that takes CO2 and creates usable, sellable propane — all at a relatively low cost considering the technology involved. In a press release concerning the research, Asadi reiterated the importance of making renewable chemical manufacturing: “It’s the best way to close the carbon cycle without losing the chemicals we currently use daily.”
Wondering how it works? The electrolyzer’s unique catalytic system uses inexpensive, readily available materials to produce tri-carbon molecules — fundamental building blocks for fuels like propane.
To ensure a deep understanding of the catalyst’s operations, the team employed a combination of experimental and computational methods. This rigorous approach illuminated the crucial elements influencing the catalyst’s reaction activity, selectivity and stability. A distinctive feature of this technology, lending to its commercial viability, is the implementation of a flow electrolyzer. This design permits continuous propane production, sidestepping the pitfalls of the more conventional batch processing methods.
“Designing and engineering this laboratory-scale flow electrolyzer prototype has demonstrated Illinois Tech’s commitment to creating innovative technologies. Optimizing and scaling up this prototype will be an important step toward producing a sustainable, economically viable and energy-efficient carbon capture and utilization process,” says Advanced Research Projects Agency-Energy Program Director Jack Lewnard.
This innovation is not Asadi’s first venture into sustainable energy. He previously adapted a version of this catalyst to produce ethanol by harnessing carbon dioxide from industrial waste gas. Recognizing the potential of the green propane technology, Asadi has collaborated with global propane distributor SHV Energy to further scale and disseminate the system.
“This is an exciting development, which opens up a new e-fuel pathway to on-purpose propane production for the benefit of global users of this essential fuel,” said Keith Simons, head of research and development for sustainable fuels at SHV Energy.
Illinois Tech Duchossois Leadership Professor and Professor of Physics Carlo Segre, University of Pennsylvania Professor of Materials Science and Engineering Andrew Rappe, and University of Illinois Chicago Professor Reza Shahbazian-Yassar contributed to this work. Mohammadreza Esmaeilirad (Ph.D. CHE ’22) was a lead author on the paper.
Below, hear more from Professor Asadi on his work and the implications of this research for the propane industry at large.
Can you briefly explain how the electrolyzer works?
The driving force for the catalytic reaction is electricity. From there, it can use that force to create chemicals — propane in this case. It’s a relatively simple process of using gas/liquid/solid interface through chemistry (a catalytic reaction). It could be used to convert other fuels besides propane, but our research tells us that propane, or C3+ products, is one of the most economically feasible byproducts of this innovation. [Editor’s note: Professor Asadi started his research on this topic in 2012.]
How inexpensive are we talking? How does it compare to traditional costs to produce/transport propane?
Absolutely — the overall goal is to make the renewable liquefied petroleum gas (LPG) in the market range that consumers are used to paying for conventional propane. I know this varies by season as well as demand and supply, but that gives us an opportunity to level out the gaps in the market, too, to some extent. Currently, because of the newness of the technology, we are on the upper limit of the market price. But this is without considering the government incentives available for producing and selling renewable fuels. There are some challenges we are still working to resolve as we move forward and try to get the tech to market, and one of those is scalability for larger operations.
On that note, you mentioned you have partnered with SHV to scale and disseminate the technology. What does this involve at this point?
The partnership with SHV Energy helps us get the research to the market in a way that is viable for producers to see a return on investment and a feasible way to grow the production using the tech. Additionally, for renewable fuels and chemicals, there is instant government incentives — either through tax cuts or other funding. SHV is helping in this arena as well and is how we were originally connected (through an ongoing project with the Department of Energy). The main goal of the partnership is to take the research we’ve done (and the tech that was created from it) and help Illinois Tech take it to the point of commercialization.
What are the current challenges to mass adoption for the electrolyzer?
Currently, the biggest challenge is with supply chain issues and concerns. To scale this tech up significantly, it needs a lot of materials involved. Determining the best way to scale this model and ensure all safety challenges are considered will be the next big steps in getting the tech to mass adoption. One good thing about the technology is that through the use of renewable electricity in the production of the fuel, we further the end goal of using all renewables for this production. But that also means working toward cleaner electricity production in the U.S. as well.
Disclaimer: “Research reported in this publication was supported by the National Science Foundation under Award Number 2135173, the Advanced Research Projects Agency-Energy under Award Number DE-AR0001581, and SHV Energy. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation, the Advanced Research Projects Agency-Energy, or SHV Energy.”
Mohammad Asadi, “Imidazolium Functionalized Transition Metal Phosphide Catalysts for Electrochemical Carbon Dioxide Conversion to Ethanol,” National Science Foundation; Award Number 2135173
Mohammad Asadi, “Direct Conversion of Flue Gas to Value-Added Chemicals Using a Carbon-Neutral Process,” Advanced Research Projects Agency-Energy; Award Number DE-AR0001581


